Golf and Corrective Action
 Imagine that you're on a golf course. As you line up your shot and swing your club… your goal is to score at or below “par”. “Par” is the maximum number of strokes an expert golfer is allowed to take for each individual hole or to complete all the holes on a golf course.
Imagine that you're on a golf course. As you line up your shot and swing your club… your goal is to score at or below “par”. “Par” is the maximum number of strokes an expert golfer is allowed to take for each individual hole or to complete all the holes on a golf course.
Now imagine that every time you didn't achieve par or below, you were issued a “Corrective Action Request”. In other words, you would have to identify and eliminate the “root cause” of why you failed to achieve par or below. And if it happens again, you get another “Corrective Action Request” citing your corrective action system for being ineffective!
You review your past golf scores - seeking one or more “root causes” preventing you from achieving a score at or below “par”. While you notice there are days where you score better or worse, you generally score within a particular range. That's because EVERY process has some degree of natural variation… and what you're observing is the NORMAL “process variation” inherent in YOU as a golfer.
Unable to identify a single “root cause”… or even a combination of “root causes” that you could address and suddenly begin achieving a score at or below “par”, you are able to identify several actions you can take to improve your score (e.g., more diligent practice, buying new clubs “fitted” specifically to you, getting a golf coach, working out to build greater upper body strength, learning how to better gauge the wind).
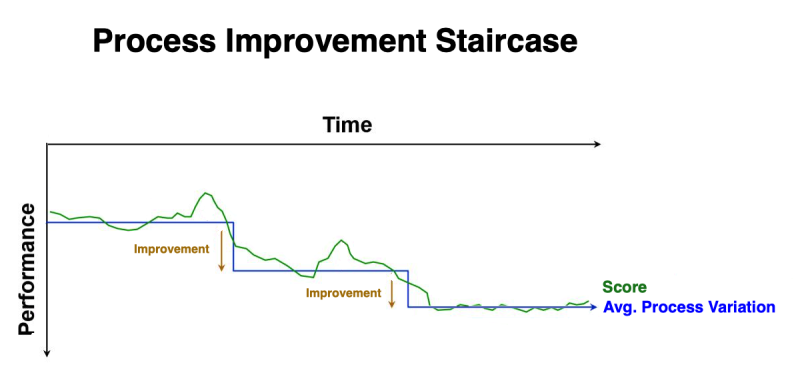
As you implement each of these actions, you notice that your scores improve to a point, and then appear to plateau. That's because these improvements are changing the natural “process variation” inherent in YOU as a golfer. And each new improvement results in a “new normal”. In a graph, rather than a linear trend, your improvements look much like a staircase. However, at some point, your scores will plateau due to circumstances beyond your control… such as weather conditions (e.g., temperature, humidity, wind), the consistency of the green, the length of the grass (on that specific day), your physical condition and ability to concentrate on that specific day. All of these circumstances are the “common cause” variations with the game of golf.
While golf was used in the above example, the analogy could be any sport involving accuracy and precision… or even business processes!
In the Golf example, “Par” was the “Objective” - and the number of strokes was the KPI (Key Performance Indicator). In a production process, the KPI could be the number of defects produced… with a goal of reducing that number.
No matter how much management encourages, motivates, or incentivizes production personnel to do better… their hands are tied by the limitations inherent within the production process. And because “common cause” variations have NO assignable root cause, there is no way to implement an effective corrective action!
The ONLY way to reduce common cause variation is through significant changes to the process (e.g., re-engineering).
"Risk Management System" instead of "Corrective Action System"?
| Common Cause | normal / usual (expected), quantifiable, random variation with NO assignable root cause |
| Assignable Cause | abnormal / unusual (unexpected), not previously observed, non-quantifiable variation WITH an assignable root cause |
There are two types of variation in every process… “Common Cause” (normal/usual) and “Assignable Cause” (abnormal/unusual).1) Ignoring these concepts, traditional quality management systems have historically addressed every nonconformity through a “corrective action system” - without considering whether it was the result of a “Common Cause” or “Assignable Cause” variation. And today, many quality management system standards continue to perpetuate this flawed approach.
<note>For no reason that I've been able to ascertain, W. Edwards Deming preferred using the term “Special Cause” rather than “Assignable Cause”.2)</note>
In order to differentiate nonconformities resulting from “Common Cause” and “Assignable Cause” variations, wouldn't it make more sense to address each nonconformity through a “risk management” system rather than a “corrective action” system?
By definition, a “corrective action” must “eliminate the cause of a detected nonconformity or other undesirable situation”.3) Therefore, in order to properly implement a “corrective action”, there must be an assignable “root cause”. And in order for there to be an assignable “root cause”, the nonconforming condition must be the result of an “Assignable cause” variation (i.e., an “unnatural pattern” in the process, which is unusual, not previously observed, non-quantifiable variation in the process). This means that where the risk of a recurrent nonconformity is eliminated - through a risk control “Action” (i.e., reducing either the likelihood/probability and/or the consequence/impact to Zero) - so has the root cause of any nonconformities (or other undesirable situation) that the risk had produced. This type of “reactive” risk elimination is actually corrective action.
“Common cause” variations are inherent to every existing process… with no “assignable” root cause. Therefore, it would be impossible to eliminate these variations. However, “common cause” variations can be mitigated through applying controls (i.e., reducing the likelihood/probability and/or the impact of consequences).
| Common (Random) Causes | Assignable (Special) Causes |
|---|---|
| Inadequate (poor) Training and/or ineffective verification of competence | Incorrect operation of equipment (Consider “error-proofing”) |
| Ambiguous/incomplete Instructions or Procedures | Inconsistent execution of process(es) |
| Inadequate (poor) Maintenance | Equipment Malfunction |
| Inconsistent Raw Material | Use of incorrect (wrong) raw material |
| Inadequate Measuring Instrumentation (lacking required accuracy & precision) | Instrument Out-of-Tolerance condition impacts product |
Examples of common “Risk Controls” include, but are not limited to:
- Revised/Updated training material(s) and/or enhanced training methods
- New employees work under direct supervision until deemed competent by Supervisor
- Revised/Updated (more accurate/comprehensive) procedures or methods
- Implementation of a more comprehensive equipment maintenance program
- New/Updated equipment (e.g., with improved accuracy, precision, reliability)
- Change the process (e.g., incorporating new technologies)
- Increasing measurement accuracy ratios of M&TE to product tolerances (to reduce the risk of measurement errors)
- Improving “detection” of nonconformities through adding Checks/Reviews (e.g., by humans of Automated Optical Inspection)
- Obtaining a “waiver” from the customer to change/modify or eliminate a requirement
Significant reductions in “common cause” variations may be realized through “modifying” or “re-designing” the existing processes.
Conclusion
How much more would your quality improve if you replaced your “corrective action” system with a much more effective and encompassing “risk management” system?
IF a nonconformity is due to a “Common Cause” variation, then “controls” could be put in place to “mitigate” the risk of the nonconformity recurring. Alternatively, IF a nonconformity is determined due to an “Assignable Cause” variation, then the process can be changed to “eliminate” the cause (or risk) of the nonconformity recurring.
Such a system would keep all of the problem solving and root cause analysis tools (e.g., the 5 Whys, Fishbone Charts, Fault Tree Analysis, Apollo Root Cause Analysis), while using the “root cause theories” as input to a "Failure Mode and Effects Analysis" (FMEA)… or PFMEA (“Process Failure Mode and Effects Analysis”).
Upon completing a PFMEA, one quickly realizes just how few “true” corrective actions are actually possible. And that by shifting the mindset to “risk management”, rather than being obsessed with “risk elimination” (aka “Corrective Action”), significant quality improvements can finally be realized.